Abstract
Background: CD79b is expressed by nearly all B-NHL. DCDS0780A is a THIOMABä antibody drug conjugate consisting of the potent anti-mitotic agent, monomethyl auristatin E (MMAE), conjugated to a humanized IgG1 anti-human CD79b monoclonal antibody via a protease-labile linker. THIOMABTMtechnology allows an essentially homogeneous product from the conjugation of two drugs per antibody to engineered cysteine residues. We conducted a Phase I study to assess the safety, tolerability, recommended Phase II dose (RP2D), pharmacokinetics (PK), and biologic activity of DCDS0780A alone or in combination with rituximab in pts with relapsed/refractory B-NHL.
Methods: Pts received DCDS0780A (0.3-4.8 mg/kg) intravenously every 21 days until disease progression or unacceptable toxicity in a standard 3+3 design. Dose limiting toxicities (DLT) were assessed in Cycle 1. Additional pts were enrolled at clinically active doses to further evaluate safety and efficacy based on Lugano Classification starting 6-12 weeks after first dose and every 3 months thereafter. Response assessment included PET for DLBCL pts.
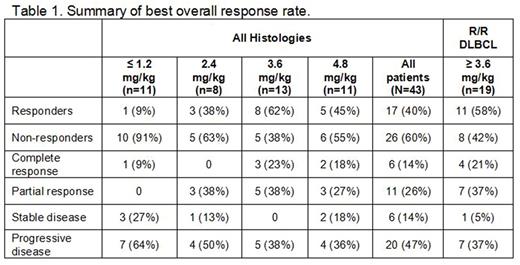
Results: As of May 31, 2017, 48 pts have been enrolled with median age of 67 years (range 32-86). Pts had diffuse large B-NHL (DLBCL, n=32), follicular lymphoma (n=11), mantle cell lymphoma (n=1), or marginal zone lymphoma (n=4). Pts were heavily pre-treated: 32 had ≥ 3 prior regimens, all had received prior rituximab, and 3 had received autologous stem cell transplantation. Of 43 pts with available response assessment for last therapy prior to study drug, 25 (58%) were refractory, defined as no response to treatment. Pts received a median of 3 doses (range 1-19) of single agent DCDS0780A. The protocol-specified MTD was not formally reached; however, 4.8 mg/kg DCDS0780A was determined to be the RP2D based on the overall safety and tolerability profile at that dose. The most common treatment-emergent adverse events (AE) occurring in ≥ 15% of pts were fatigue (42%), nausea (38%), diarrhea (30%), abdominal pain (25%), neutropenia (23%), vision blurred (23%), corneal deposits (19%), decreased appetite (19%), and headache (17%). Grade ≥ 3 AEs in ≥ 2 pts included neutropenia or decreased neutrophil count (23%), decreased white blood cell count (6%), thrombocytopenia (6%), and hypercalcemia (4%). One DLT of Grade 4 systemic inflammatory response syndrome (SIRS) occurred at 2.4 mg/kg. Nine pts discontinued treatment due to AE, including ocular events in 5 (corneal deposits in 2; corneal opacity, keratitis, and blurred vision in 1 each), and hypoxic-ischemic encephalopathy, hypercalcemia, muscular weakness, and acute respiratory distress syndrome (all n=1). Four pts had dose reductions due to AE, including Grade 3 thrombocytopenia (n=1) and Grade 2 blurred vision (n=3, 1 also with Grade 2 corneal deposits). Two deaths were reported within 30 days of last treatment; 1 due to anoxic encephalopathy following SIRS and 1 due to PD. Ocular events considered related to DCDS0780A, mostly consisting of blurry vision with corneal deposits or keratitis, were observed in 15 pts (13/15 pts at ≥ 3.6 mg/kg), all but 2 of which were Grade 1 or 2. Ocular findings and symptoms resolved in most pts with dose hold and topical intervention; 3 pts underwent retreatment at a lower dose without recurrence. Exposures (Cmax and AUC) of the acMMAE and total antibody analytes increased with an increase in dose (0.3 - 3.6 mg/kg), following DCDS0780A administration in Cycle 1. Clearance values for acMMAE and total antibody analytes were similar at clinical doses tested (0.3 - 3.6 mg/kg), with an evidence of linear PK at doses ≥ 1.2 mg/kg. Unconjugated MMAE exposures were low and demonstrated formation rate-limited kinetics. Of 43 response-evaluable pts, 17 experienced an objective response (40%), including 6 complete responses (14%) and 11 partial responses (26%) (Table 1). All responses except 1 occurred at ≥ 2.4 mg/kg; 4 pts currently on therapy have not yet undergone response assessment. The 17 responders had a median post-response on-treatment time of 2.4 months, in which 4 responders experienced disease progression, and median DOR has not yet been reached.
Conclusions: DCDS0780A demonstrates an acceptable toxicity profile and encouraging dose-related anti-tumor activity in heavily pretreated pts with relapsed/refractory B-NHL including R/R DLBCL. Updated clinical data will be presented, including RP2D in combination with rituximab.
Herrera: Pharmacyclics: Consultancy, Research Funding; Immune Design: Research Funding; Genentech: Consultancy, Research Funding; Seattle Genetics: Research Funding; Merck: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Patel: Medivation: Speakers Bureau; BMS: Speakers Bureau; Exelixis: Speakers Bureau; Genentech: Speakers Bureau; Gilead: Speakers Bureau. Burke: Celgene: Consultancy; Incyte: Consultancy; Bayer: Consultancy; Gilead: Consultancy; Genentech: Consultancy. Advani: Agensys: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Bayer Healthcare Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Sutro: Consultancy; Nanostring: Consultancy; Juno Therapeutics: Consultancy; FortySeven: Research Funding; Gilead: Consultancy; Janssen: Research Funding; Regeneron: Research Funding; Cell Medica: Research Funding; Genentech: Research Funding; Pharmacyclics: Consultancy; Celgene: Research Funding; Spectrum: Consultancy; Kura: Research Funding; Infinity: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Millennium: Research Funding. Cheson: AbbVie, Roche-Genentech, Pharmacyclics, Acerta: Consultancy; Acerta, Pharmacyclics, Epizyme, Gilead, Roche, AbbVi: Other: Institution receives research support . Sharman: Novartis: Research Funding; Genentech: Research Funding; Gilead Sciences, Inc.: Consultancy, Honoraria, Research Funding, Speakers Bureau. Penuel: Genentech, Inc.: Employment. Polson: genentech: Employment. Leng: genentech: Employment. Li: genentech: Employment. Schuth: genentech: Employment. Vaze: genentech: Employment. Samineni: genentech: Employment. Elstrom: genentech: Employment. Diefenbach: Genentech: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal